M1D19
Project achieved:
- CE; compliant with all EU directives
- ISO 13485 certification
- FDA Registered
- 5-Year Warranty Coverage
- 30-Day (Risk-Free) Returns
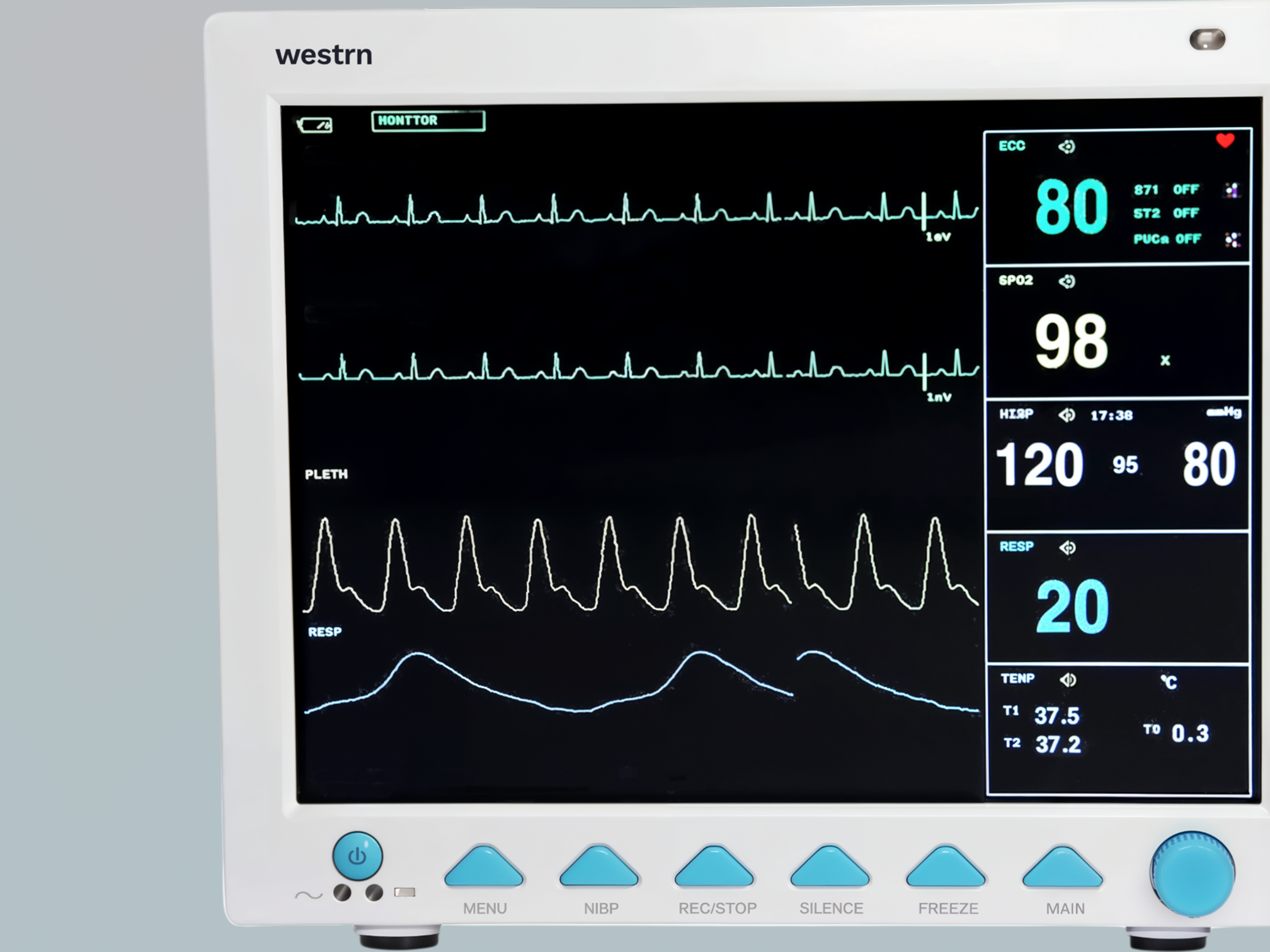
Multi-parameter bedside monitor for continuous patient vital assessment.
The M1D19 Patient Monitor is a versatile, high-performance monitoring system designed for adult, pediatric, and neonatal care. Featuring a 12.1” color TFT LCD display, it shows up to eight real-time waveforms and key parameters including ECG, RESP, NIBP, SpO₂, TEMP, and pulse rate, with optional upgrades for IBP and EtCO₂. Ideal for critical care, emergency, or post-op settings, it offers arrhythmia detection, ST-segment analysis, and plethysmogram tracking for accurate diagnostics. Compact and portable, the M1D19 supports data storage, wired/wireless networking, and an optional built-in printer, with a user-replaceable battery for continuous monitoring during transport—delivering reliability and adaptability in fast-paced clinical environments.
-
Comprehensive multi-parameter monitoring for adults, pediatrics, and neonates.
-
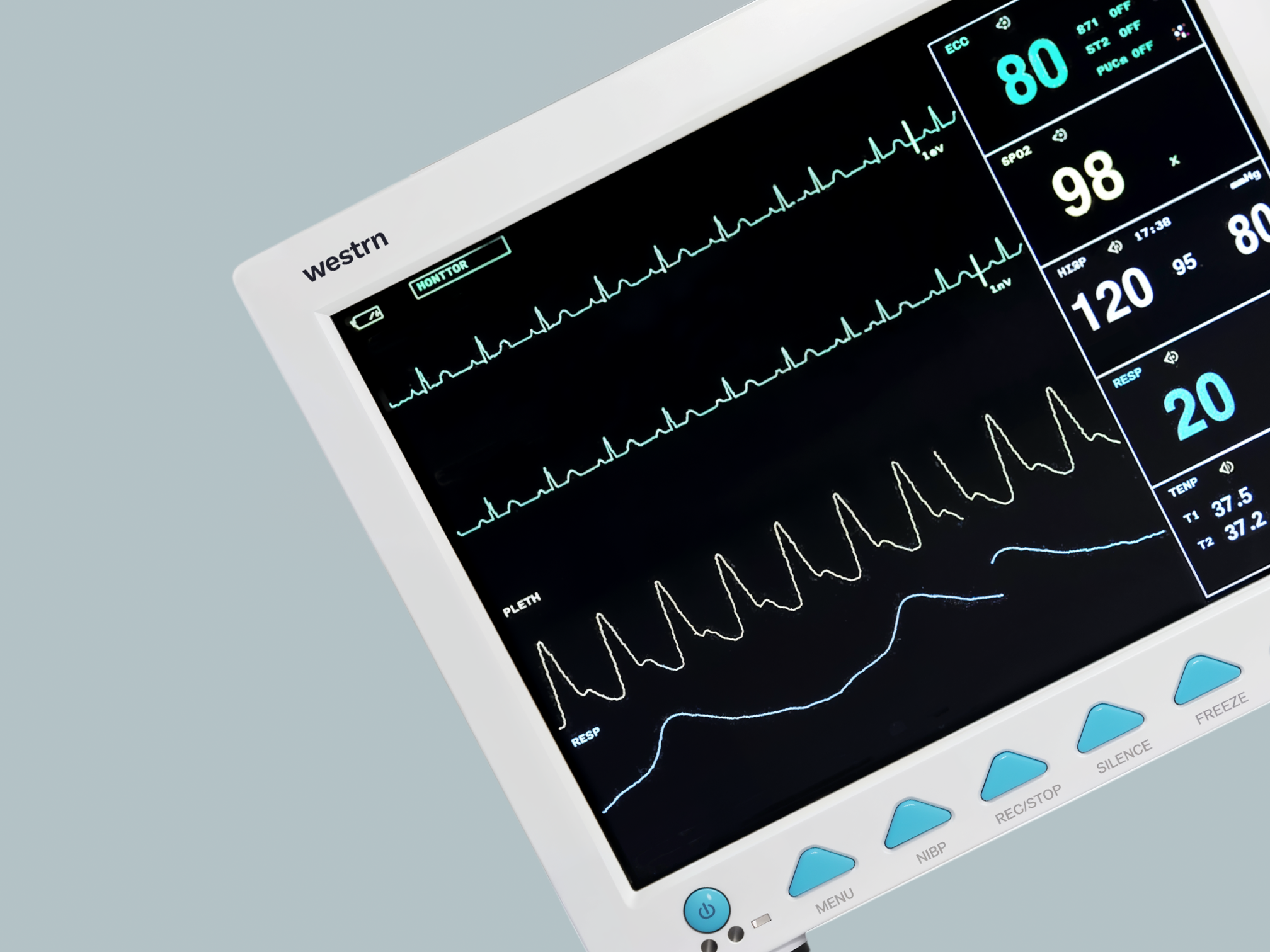
12.1" color TFT screen displays eight real-time waveforms and vital signs simultaneously.
-
Modular integration combines ECG, SpO2, NIBP, RESP, TEMP, and optional IBP/CO2 in one unit.

-
Flexible connectivity via wired or wireless link to central monitoring systems.

-
Built-in battery enables mobility and uninterrupted monitoring during patient transport.

-
Advanced features like ST analysis, drug dose calculation, and trend data storage enhance care.

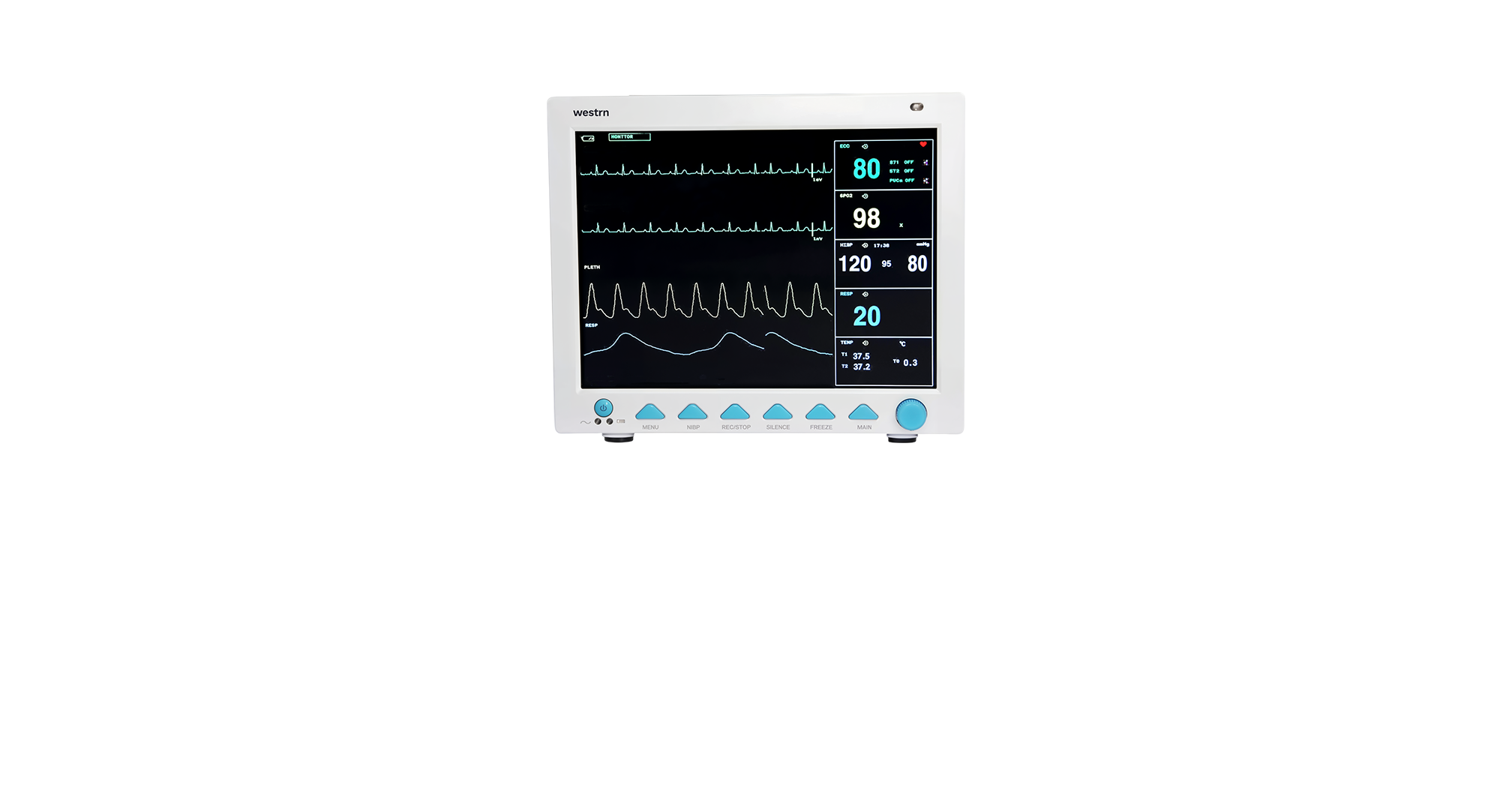
11.81” (L) x 10.63” (D) x 10.63” (H)
7.72 lbs
Specifications
M1D19
| Display | 12.1-inch color TFT LCD with real-time data and waveform display |
|---|---|
| Waveform Display | Simultaneous display of up to 8 waveforms |
| Power Supply | 100–120V~, 50/60Hz |
| Thermal Recorder | Optional 1.89 inch built-in thermal printer |
| Networking | Supports wired and wireless connection to a central monitoring system |
| Portability | Compact, integrated design with built-in replaceable battery for mobility |
| Patient Types | Supports adult, pediatric, and neonatal patients |
| Parameter Configuration | User-selectable based on clinical requirements |
| Standard Monitoring Parameters | |
| ECG (Electrocardiogram) | Heart Rate (HR), ECG Waveform, Arrhythmia Analysis, ST-Segment Analysis |
| RESP (Respiration) | Respiration Rate (RR), Respiration Waveform |
| SpO₂ (Oxygen Saturation) | SpO₂%, Plethysmogram (PLETH), Pulse Rate (PR), Bar Graph |
| NIBP (Non-Invasive Blood Pressure) | Systolic (SYS), Diastolic (DIA), Mean (MEAN) Pressure |
| TEMP (Temperature) | Dual-Channel: T1, T2, and TD (temperature difference) |
| Optional Monitoring Parameters | |
| IBP (Invasive Blood Pressure) | CH1 & CH2: Systolic (SYS), Diastolic (DIA), Mean Arterial Pressure (MAP); IBP Waveform |
| CO₂ (Capnography) | EtCO₂ (End-tidal CO₂), InsCO₂ (Inspired Minimum CO₂), AwRR (Airway Respiration Rate) |
| Additional Functionalities | |
| Alarms | Visual and audible alarms for all monitored parameters |
| Trend Data | Storage and output of trend data |
| NIBP Measurement | Scheduled or manual non-invasive blood pressure checks |
| Alarm Event Marking | Automatically marks significant alarm events |
| Drug Dose Calculation | Calculates drug concentrations based on input parameters |



